Check out what’s clicking on FoxBusiness.com.
A popular brand of eye drops is being pulled across the country due to possible contamination that may cause visual impairment, according to the Food and Drug Administration (FDA).
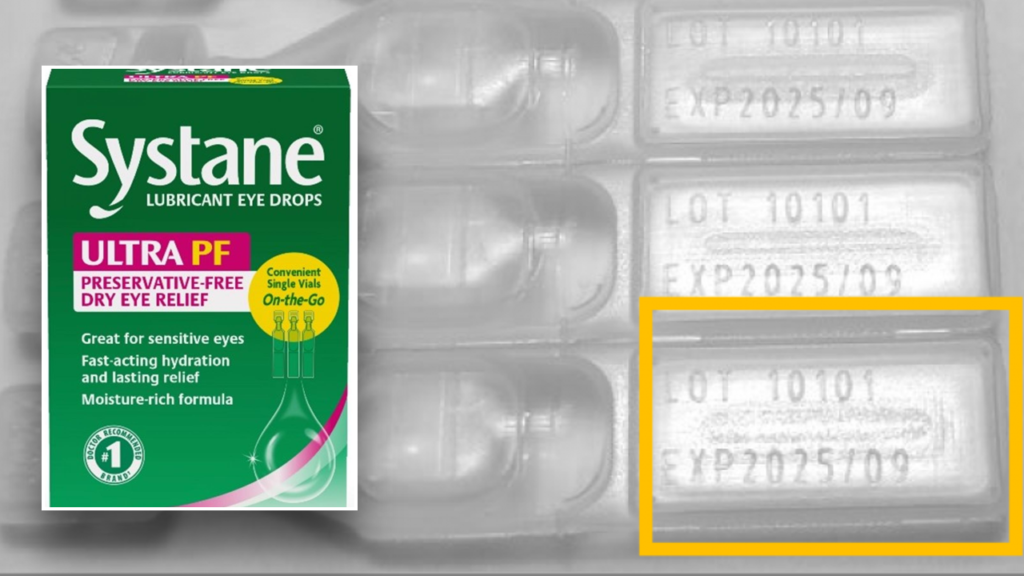
On Monday, the FDA announced that Texas-based Alcon Laboratories is voluntarily recalling a single batch of Systane Lubricant Eye Drops Ultra SPF, Single Vials On-the-Go because the products may be contaminated with fungus.
The company reported a consumer complaint that a “foreign material” was found in a sealed single-use vial and described the material as “fungal in nature.”
Fungal contamination in eye products is known to potentially cause eye infections, FDA said.
EYE PRODUCTS SOLD AT WALMART, CVS MAY POSSE RISK OF INFECTION

The Food and Drug Administration (FDA) announced Monday that Texas-based Alcon Laboratories is voluntarily recalling a single batch of “Systane Lubricant Eye Drops Ultra SPF, Single Vials On-the-Go” because the products may be contaminated with fun (Food and Drug Administration (FDA) / Fox News)
If infection occurs, the FDA said it can be vision-threatening and, in very rare cases, potentially life-threatening in immunocompromised patients.
To date, the FDA said Alcon Laboratories has not received any reports of adverse events related to this recall.
The FDA says the affected product includes Systane Lubricant Eye Drops Ultra PF, Single Vials On-the-Go, 25 count and that it is limited to lot number 10101, expiration date 2025/09.
The product can be identified by the green and pink carton design, the presence of the “Systane” and “ULTRA PF” branding on the front of the carton, and the “25 vial” package size, the FDA explained in a press release.
FDA UPGRADES RECALL OF COSTCO EGGS TO HIGHEST RISK LEVEL DUE TO SALMONELLA FEAR

“Systane Lubricant Eye Drops Ultra SPF, Single Vials On-the-Go” were recalled because the products may be contaminated with fungus, according to the FDA. (Food and Drug Administration (FDA) / Fox News)
Much of the potentially affected eye drops were also distributed nationwide to retail outlets and online stores.
Consumers who have the recalled eye drops are urged to stop using them immediately and return them to the place of purchase for a replacement or refund, the FDA said.
Distributors or retailers who have the recalled eye drops are also being urged to dispose of any remaining stock of the tainted product.
Alcon Laboratories will also notify its distributors and customers by letter, email and/or telephone call and arrange for replacement of any recalled products.

Alcon Laboratories issued the recall after a consumer complained of a “foreign material” they found in a sealed vial of eye drops. (Food and Drug Administration (FDA) / Fox News)
This latest recall comes after several eye products over the past year have been pulled from shelves due to the potential risk of infection.
In February, the eye ointment products sold at CVS and Walmart stores nationwide were recalled after the FDA found a “lack of assurance of sterility” at the manufacturing facility.

Alcon Laboratories in Texas is voluntarily recalling Systane Lubricant Eye Drops Ultra PF after a consumer complaint of foreign material being observed in a sealed vial. (Food and Drug Administration (FDA) / Fox News Latino)
The four affected products, which are intended to be sterile, are sold under the brand names Equate, CVS Health and AACE Pharmaceuticals and have expiration dates ranging from February 2024. until September 2025 The products were distributed nationwide to wholesalers, retailers and through a product distributor, Walmart, CVS and AACE Pharmaceuticals Inc.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
In November 2023 Kilitch Healthcare India Limited voluntarily recalled 27 eye drops, all of which were on the FDA’s current list of products that may be contaminated with bacteria that pose a risk of eye infection and vision loss.
None of the aforementioned recalls were linked to the outbreak of antibiotic-resistant bacteria pseudomonas aeruginosa related to eye products from Global Pharma Healthcare.
Fox News Digital’s Daniela Genovese contributed to this report.
2024-12-26 02:22:41
https://a57.foxnews.com/static.foxbusiness.com/foxbusiness.com/content/uploads/2024/12/0/0/fda-recalls-popular-brand-of-eye-drops.png?ve=1&tl=1